What's in a neuron?
How single-cell technology is revolutionizing neurodegeneration research

Neurodegenerative diseases affect more people worldwide than cancer. Yet even though scientists have been studying these diseases for decades, understanding their underlying neurobiology remains a challenge.
The most common neurodegenerative diseases include Alzheimer's and Parkinson's.
Alzheimer’s disease slowly destroys memory and thinking skills.
Parkinson’s disease causes unintended or uncontrollable movements, such as shaking, stiffness, and difficulty with balance and coordination.
Both diseases are progressive, meaning they get worse over time. There is currently no cure for these devastating neurodegenerative disorders.
Toxic build-up of specific proteins called α-synuclein and tau inside and between cells are the classic neuropathological hallmarks of several neurodegenerative diseases, including Parkinson’s and Alzheimer’s.
Existing neuropathological and clinical evidence tells us that α-synuclein and tau mutations are region and cell-type specific. In other words, some types of neurons (nerve cells) and brain areas are more affected by these toxic proteins than others.
However, it is not yet known why this is the case. In the heart of Europe - at the VIB-KU Leuven Center for Brain & Disease Research - several labs are working hard to understand this.
Pictured below: Neuroscientists working at the VIB-KU Center for Brain & Disease Research.
The VIB-KU Leuven Center for Brain & Disease Research in Leuven, Belgium.
The VIB-KU Leuven Center for Brain & Disease Research in Leuven, Belgium.
The VIB-KU Leuven Center for Brain & Disease Research in Leuven, Belgium.
The VIB-KU Leuven Center for Brain & Disease Research in Leuven, Belgium.
The VIB-KU Leuven Center for Brain & Disease Research in Leuven, Belgium.
The VIB-KU Leuven Center for Brain & Disease Research in Leuven, Belgium.






Why are certain cells and brain regions more affected in neurodegenerative diseases than others? This question - referred to as selective vulnerability - has been baffling scientists for over 100 years.
Selective vulnerability is a fundamental feature of these neurodegenerative diseases, in which different neurons (brain cells) lie on a spectrum of susceptibility to decay.
Selective vulnerability at the network level (i.e. in brain regions) has been extensively explored in both Alzheimer's and Parkinson's.
However, little is known about the mechanisms underlying selective vulnerability at the cellular level.
If scientists could figure out why and how α-synuclein and tau affect different types of neurons, they could start to understand the mechanisms underlying why some cells are more resilient to these toxic proteins.
This could, in turn, provide insight into disease mechanisms and lead to therapeutic strategies to tackle neurodegenerative disorders.

Neurons in the Brain, drawn by Lena (age 13) the daughter of Patrik Verstreken, Scientific Director and Group Leader at the VIB-KU Leuven Center for Brain & Disease Research.
Neurons in the Brain, drawn by Lena (age 13) the daughter of Patrik Verstreken, Scientific Director and Group Leader at the VIB-KU Leuven Center for Brain & Disease Research.
Enter Roman Praschberger. As far back as he can remember, Roman was questioning the world around him. This led him to study philosophy and medicine in his hometown of Innsbruck, Austria.
He then did his PhD at the UCL Queen Square Institute of Neurology in London, UK. During this time, Roman met Patrik Verstreken, Group Leader of the Laboratory of Neuronal Communication.
Now, as a postdoctoral researcher of Patrik's at the VIB-KU Leuven Center for Brain & Disease Research, Roman is channeling his curious mind into exploring how modern advances in single-cell technology can be harnessed to explore the century-old puzzle of selective vulnerability in neurodegenerative disease.
Scientists at the VIB-KU Leuven Center for Brain & Disease Research have been actively contributing to the single-cell revolution for the past few years. In particular, the Stein Aerts laboratory was a pioneer for the use of single-cell technologies.
In a interview for VIB News in 2018, Aerts explained how his fascination with single-cell technology all began: "We got really interested when two specific papers were published in the same issue of Cell in 2015, which described the use of droplet microfluidics to sequence the RNA of tens of thousands of individual cells in parallel - and more cheaply than ever. We were so excited that we called Jeroen Lammertyn, from the KU Leuven bioengineering faculty, who taught our PhD student Kristofer Davie how to design and fabricate our own microfluidic devices. Our first successful experiment came a few months later - it was a great moment to see cells cluster together as anticipated."
Thanks to single-cell technologies, Stein and his team accomplished a world first: a gene expression map of every cell in the brain of an ageing fly. The atlas is a major step towards a better understanding of human disease development.


To explain how advances in single-cell technologies can provide new insights for neurodegenerative disease research, Roman - together with colleagues Sriram Balusu, Elsa Lauwers, and research group leaders Patrik Verstreken and Bart De Strooper - recently wrote a review, published in Neuron.
(Psssst, you can read a bite-sized, easy-to-digest summary of the review here!)
In short, the single-cell revolution has given scientists incredible insight into how different brain cell types interact with disease.
New single-cell technologies such as single nucleus sequencing, soma sequencing, and spatial technologies help researchers to start understanding the complexity of neurodegenerative disorders.
However, more work is needed to understand exactly how different kinds of brain cells act in Alzheimer's and Parkinson's.
This is where Roman's research comes in.
I caught up with Roman in a lab at the VIB-KU Leuven Center for Brain & Disease Research, where I asked him to describe his latest work, published in Neuron. "In neurodegenerative diseases such as Parkinson's and Alzheimer's, some nerve cells are highly vulnerable and degenerate, while others are resilient and survive." Roman explains. "In this project, we explored the idea that these resilient neurons might be able to tell us some of nature’s own secrets. After all, these resilient neurons seem to be doing something right - so why not learn from them?" His aim is that in the future, this knowledge might be harnessed to treat the vulnerable neurons, whose loss causes debilitating neurological symptoms.
Roman Praschberger shares a bite-sized summary of his latest paper: "Neuronal identity defines α-synuclein and tau toxicity" (Praschberger et al., 2023, Neuron).
They focused specifically on the two proteins already known to be critical drivers of neurodegeneration: α-synuclein and tau. "Remarkably, even though α-synuclein and tau can be toxic to neurons in general, we can find multiple resilient neuron types that express high levels of α-synuclein and tau in human brain datasets and fruit-fly models," Roman continues. "This suggests that they instead use strategies to better deal with potentially toxic proteins."
To find out what these resilience strategies are, Roman and his colleagues compared gene expression differences between vulnerable and resilient neurons with a technique called single-cell RNA sequencing. "We found that α-synuclein and tau resilient neurons differentially regulate mitochondrial energy metabolism, Ca2+ homeostasis and synapses (i.e. contact sites between neurons). Remarkably, when we experimentally modulated genes in these pathways we found several of them to potently reduce the toxicity of abnormal tau in our fly models, confirming that resilient neurons contain very valuable insights for potential new treatments."

Roman Praschberger working in the lab at the VIB-KU Leuven Center for Brain & Disease Research.
Roman Praschberger working in the lab at the VIB-KU Leuven Center for Brain & Disease Research.
A Figure from Roman's publication that demonstrates cell-type diversity across the entire fly brain. Individual dots represent unique cells and clustered groups the different cell-types.

Suresh Poovathinghal, expert in single-cell microfluidics at the VIB-KU Leuven Center for Brain & Disease Research.
Suresh Poovathinghal, expert in single-cell microfluidics at the VIB-KU Leuven Center for Brain & Disease Research.
I sit with Suresh - head of Single-Cell Microfluidics at VIB Technologies, to find out more about what specific single-cell technology was used in this project, and why.
VIB was one of the earliest adopters of single-cell technologies. In 2016, VIB Tech Watch team invested in one of the first 10x Genomics Chromium platforms in Europe, a ground-breaking technology that enables large scale single-cell RNA sequencing. However, applying these commercial setups to large scale single cell profiling has been far too expensive.
To solve this problem, Suresh worked with other researchers in the center (including Stein Aerts, who we introduced earlier!) to develop HyDrop, a powerful yet affordable droplet microfluidics platform.
"In projects like Roman's - where we're dealing with small fly cells that have low amounts of RNA transcripts - it's important to have really high encapsulation rates. The more cells you can efficiently capture, the higher chance of getting more diverse dataset," Suresh explains, before diving into the technical details of the project.
"We used multiple sequencing techniques for single cells, and bulk sequencing workflows were used to understand the underlying cellular diversity of fly brain. For high-throughput single-cell profiling, 10X Genomics’ 3’ Whole Transcriptome Analysis and for bulk sequencing of limited FACS sorted cell population, modified version of SMART seq 3 workflow was used."
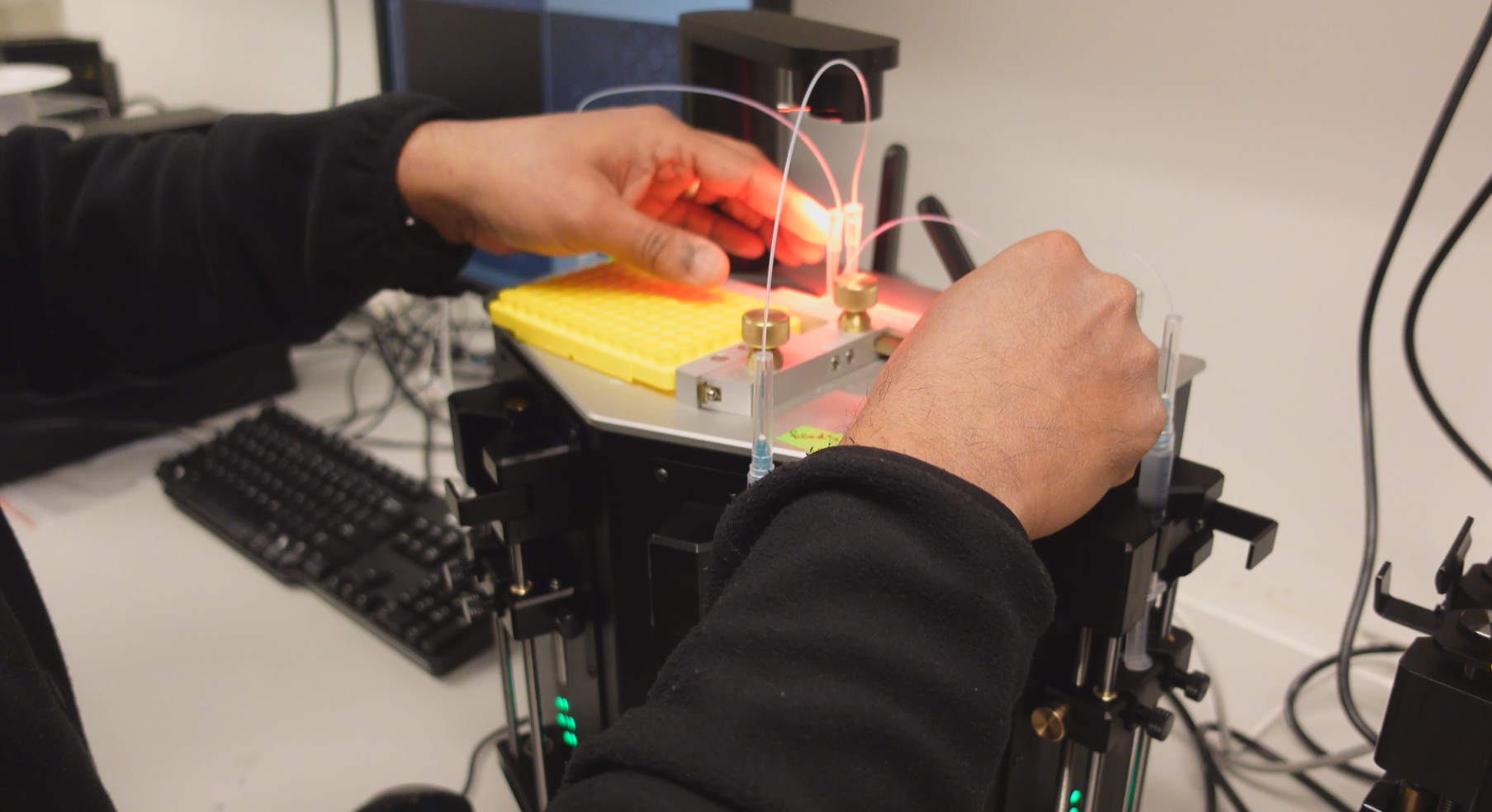
"ONYX platform is an automated syringe pump system which feeds special reagents/enzymes into a novel microfluidic droplet generating chip, which we also developed internally." Suresh explains, while showing me the device pictured.
"ONYX platform is an automated syringe pump system which feeds special reagents/enzymes into a novel microfluidic droplet generating chip, which we also developed internally." Suresh explains, while showing me the device pictured.
"What we did with Roman's project was a novel adaptation of using the HyDrops’ droplet generation chip with the 10X Genomics reagents to encapsulate single cells. We have seen that the encapsulation efficiency with the HyDrop chips on ONYX platform are far superior and reliable compared to the 10X Genomics chromium controller.”
Finally, I was curious to know what all of this means for the future of neurodegenerative disease research.
Thanks to the affordability of HyDrop, scientists are able to apply this single-cell technology to larger data sets than ever before. "We are constantly working toward improving the performance of HyDrop," Suresh shares. "This is with the hope that we can do large-scale single-cell and nuclei sequencing in a affordable manner which would further facilitate new insights to cellular mechanisms underlying disease."
I ask Roman for his thoughts on the future translational potential of his research. “Our study shows that some nerve cells are better prepared than others to deal with toxic α-synuclein or tau," Roman concludes. "What excites me most is that in the future we might be able to leverage these resilient neurons’ strategies as nature’s own template to find new treatments for α-synuclein and tauopathies."
Scrollyboard by Bethan Burnside (Neuroscience Communicator at VIB-KU Leuven Center for Brain & Disease Research)
